The Structure of Metal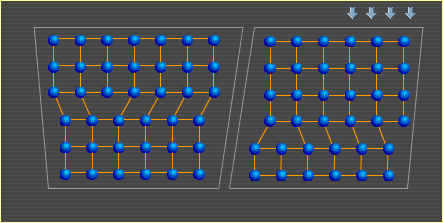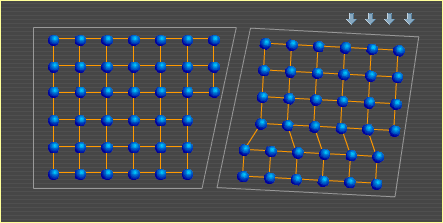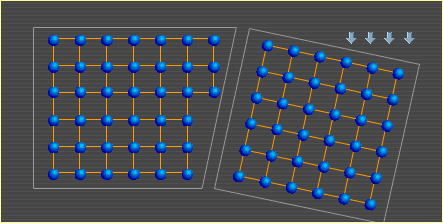
A metal is comprised of many individual crystals, or grains. These crystals are held together by electrons in the same way that electrons hold the atoms within each crystal together. For more on this type of bond, see Metal Basics. An important characteristic of metal is its ability to bend. Here are two metal crystals side by side. Notice how their atoms shift to produce a bend. This is a simplified example that shows how crystals can change shape to allow metal to bend. The crystal on the left deforms as shown in Slip, but in the opposite direction since the force is being applied to it by the rotating crystal on the right. A similar slip event in the right crystal then allows further change to the overall shape. In a real metal, crystals contain many more atoms and dislocations (defects in the otherwise orderly structure), and they are surrounded by many other crystals that can also alter their shapes. Also, although the crystals above are depicted in two dimensions, imagine that there are many layers of similarly arranged atoms behind this top layer, creating a three-dimensional, cubic structure. To continue to the next section, select Heat. Metal Basics | Move | Slip | Bend | Heat |
|
|
|
|
|
Building on Ground Zero Home | Send Feedback | Image Credits | Support NOVA |
© | Created August 2006 |

