
|

|
Eating It (as in Yum) and Eating It (as in Bye-Bye) Back to The Dating Game So is everything you eat really radioactive? You bet. Should you worry about this? Nah. 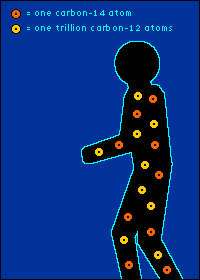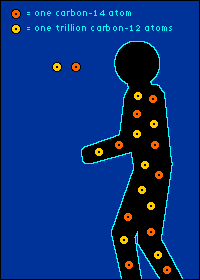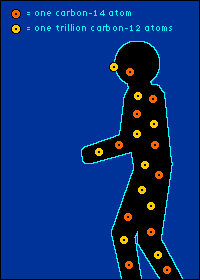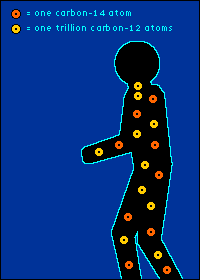 The carbon-14 created by cosmic radiation makes up only a
fraction of the carbon in our atmosphere. But it is there, and
just like carbon-12, it can be taken in by a growing plant and
become a part of that plant. (As you may know, plants take in
carbon dioxide, or CO2, separate the carbon from the oxygen, then release the
oxygen back into the atmosphere.)
The carbon-14 created by cosmic radiation makes up only a
fraction of the carbon in our atmosphere. But it is there, and
just like carbon-12, it can be taken in by a growing plant and
become a part of that plant. (As you may know, plants take in
carbon dioxide, or CO2, separate the carbon from the oxygen, then release the
oxygen back into the atmosphere.) So every plant contains a certain percentage of carbon-14. And so do those things that eat plants. And so do those things that eat the things that eat plants. The percentage of carbon-14 in all of these living things is the same as the percentage of carbon-14 in the atmosphere. At least it's the same while they're living. When a plant or animal dies, no carbon (in any form) can enter its system to become a part of it. Now we get into the nitty-gritty of carbon dating. 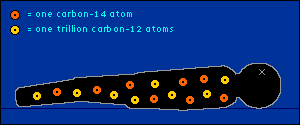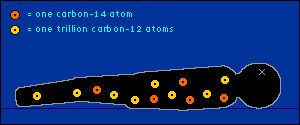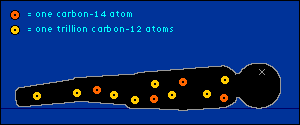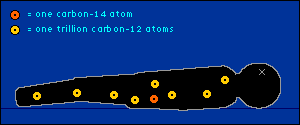 The carbon-14 within every once-living thing will someday turn
back into nitrogen-14. If we knew the amount of carbon-14 a
once-living thing had while it was alive and the rate at which
it changed (i.e., how fast it changed) back into nitrogen,
then we could figure out how long ago it lived.
The carbon-14 within every once-living thing will someday turn
back into nitrogen-14. If we knew the amount of carbon-14 a
once-living thing had while it was alive and the rate at which
it changed (i.e., how fast it changed) back into nitrogen,
then we could figure out how long ago it lived.Well, it turns out that we do know. The amount of carbon-14 in the atmosphere (and therefore in living things) has not changed all that much over time. And we do know the rate at which carbon-14 changes back to nitrogen-14, though what this rate is is not as straightforward as it could be. Thus we move on to the topic of half-life. Next: (My So Called) Half-Life (Shockwave version) Non-Shockwave version Does Race Exist? | Meet Kennewick Man Claims for the Remains | The Dating Game | Resources Transcript | Site Map | Mystery of the First Americans Home Editor's Picks | Previous Sites | Join Us/E-mail | TV/Web Schedule About NOVA | Teachers | Site Map | Shop | Jobs | Search | To print PBS Online | NOVA Online | WGBH © | Updated November 2000 |