
|

|
Frequently Asked Questions
Help/Resources
|
Treatments
(provided by Susan Connors, B.S., Surgical Research
Laboratory, Children's Hospital, Boston, Massachusetts)
- What is angiogenesis?
-
What is an angiogenesis inhibitor?
-
Are angiogenesis inhibitors being used to treat
cancer? Are angiogenesis inhibitors being used alone
or in combination with other therapy?
- What is a clinical trial?
-
Who is eligible for a clinical trial for cancer?
-
Do some patients who enroll in cancer trials
receive a placebo instead of the drug?
-
How can I find out about cancer trials available to
me? How can I find out about clinical trials testing
angiogenesis inhibitors?
-
Can I use an angiogenesis inhibitor with the
chemotherapy that I am on right now?
-
Are these drugs available for compassionate use?
-
Are angiogenesis inhibitors in clinical trial for
children with cancer?
-
Can angiogenesis inhibitors be used for other
diseases?
-
How can I find out about clinical trials for
endostatin?
-
When will Phase II endostatin trials begin?
-
How can I find out about clinical trials for
angiostatin?
-
Does Dr. Folkman see patients?
|
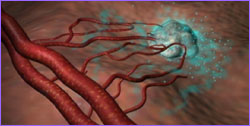 Animation of angiogenesis, with tumor calling forth
new offshoots from an existing blood vessel.
Animation of angiogenesis, with tumor calling forth
new offshoots from an existing blood vessel.
|
Q1. What is angiogenesis?
A: Angiogenesis is the process by which the body
forms new, small blood vessels called capillaries. Normally
this process is short-lived and beneficial, as in wound
healing, reproduction, and fetal development. Under some
abnormal conditions, angiogenesis can develop and be
detrimental, as it contributes to the growth of cancer and
other diseases. This abnormal angiogenesis allows tumors to
grow and metastasize (spread to other organs or other parts
of the body) by providing nutrients and growth factors
brought in by the new blood vessels. Virtually all tumors
are dependent on angiogenesis for their growth.
Q2. What is an angiogenesis inhibitor?
A: An angiogenesis inhibitor is a naturally occurring
or synthetic molecule that can interfere with or "block"
angiogenesis. Since abnormal angiogenesis rarely turns
itself off spontaneously, the goal of antiangiogenic therapy
is to stop the formation of new blood vessels and to break
up the existing abnormal blood vessels. Angiogenesis
inhibitors target new, growing blood vessels and do not
affect normal resting blood vessels in the body.
Q3. Are angiogenesis inhibitors being used to
treat cancer? Are angiogenesis inhibitors being used
alone or in combination with other therapy?
A: Angiogenesis inhibitors are a new class of drug
being studied in clinical trials in patients with advanced
cancer that has failed to respond to conventional therapy.
These inhibitors are not yet approved by the Food and Drug
Administration (FDA) for use outside of clinical trials.
Many angiogenesis inhibitors are under development and
investigation, and more than 20 different angiogenesis
inhibitors are in clinical trial for cancer at medical
centers in the United States.
 Eventually doctors may add antiangiogenesis drugs
to more traditional cancer-fighting approaches, such
as chemotherapy.
Eventually doctors may add antiangiogenesis drugs
to more traditional cancer-fighting approaches, such
as chemotherapy.
|
|
Angiogenesis inhibitors target the blood supply feeding the
tumor, while surgery, standard chemotherapy, and radiation
therapy target the tumor itself. Eventually, angiogenesis
inhibitors may be added to chemotherapy or to radiation
therapy or to other treatments such as vaccine therapy and
gene therapy. Also, in the future, combinations of
angiogenesis inhibitors may be used together.
|
 All cancer drugs, whether for conventional
chemotherapy or unconventional
angiogenesis-inhibiting treatment, must go through
clinical trials.
All cancer drugs, whether for conventional
chemotherapy or unconventional
angiogenesis-inhibiting treatment, must go through
clinical trials.
|
Q4. What is a clinical trial?
A: A clinical trial is a strictly monitored
scientific study, in which new treatments are tested on
patients in a hospital or clinic. Clinical trials determine
whether a new treatment is safe, effective, and a better
alternative to current standard therapy. All clinical trials
involving humans must be approved by the institution's
review board. This review board is made up of physicians,
administrators, scientists, ethicists, and laymen. All
patients participating in a clinical trial will go through
an informed consent process before starting treatment. This
process will include a discussion between doctor and patient
about all aspects of the investigational therapy, including
possible risks and benefits of treatment. A patient can
withdraw from a clinical trial at any point, for any reason,
even after consent is given and a consent form is signed.
Treatment found to be safe and effective can be made
available for widespread use, after approval by the FDA. New
investigational drugs are usually for patients whose disease
has not responded to current standard therapy and should not
be used as substitutes for proven effective treatment.
Clinical trials take place in three different phases:
Phase I
After extensive testing on animals in the laboratory, a
small number of patients (usually fewer than 100) are
enrolled in a Phase I clinical trial. This initial phase
of testing will determine safety, common side effects,
information about dosage, and the best way to give the
drug. At this stage, little is known about how effective
the new treatment will be.
Phase II
Phase II clinical trials continue to evaluate the safety
of the treatment and provide some information about the
effectiveness of the drug. Phase II trials include a
larger number of patients and may focus on a particular
type of cancer.
Phase III
Phase III clinical trials are conducted to confirm the
effectiveness of the drug. These studies often compare the
experimental treatment with current standard treatment or
combine the experimental treatment with standard treatment
and compare this to standard treatment alone. These trials
usually consist of two groups of patients: the "treatment
group," which is given the experimental treatment, and the
"control group," which is given the standard treatment.
Phase III trials usually enroll over 1,000 patients.
Q5. Who is eligible for a clinical trial for
cancer?
A: Most cancer centers have a number of clinical
trials available for patient participation. These clinical
trials follow a written protocol, which is a set of rules
for treatment. This protocol lists diagnostic tests,
procedures, medications, doses, length of treatment,
inclusion criteria, and exclusion criteria. A patient's
eligibility may depend on factors such as current medical
condition, extent of disease, type of tumor, and prior
therapy. A member of the study research team will review
eligibility criteria with each patient before enrollment.
Most clinical trials require that a patient's disease be
refractory (unresponsive) to conventional therapy, or be a
disease for which there is no effective known curative
therapy.
Q6. Do some patients who enroll in cancer trials
receive a placebo instead of the drug?
A: Cancer trials rarely use placebos (a dummy
treatment containing no drug). In trials that compare the
experimental treatment to the best standard treatment,
patients are usually "randomized" (chosen at random) to one
of two different groups: the "treatment group," which is
given the experimental treatment, and the "control group,"
which is given the standard treatment. In this situation,
the patient gets assigned to one group or the other and may
or may not receive the experimental therapy.
Q7. How can I find out about cancer trials
available to me? How can I find out about clinical
trials testing angiogenesis inhibitors?
A: There are many hundreds of cancer trials being
conducted in the United States and abroad. Most of the
trials are sponsored by government agencies and
pharmaceutical companies, and are conducted at cancer
centers, hospitals, and some doctor's offices. There is no
single resource that lists all cancer trials. You can ask
your oncologist about clinical trials being conducted at the
center where you are being treated. In addition, many cancer
centers and pharmaceutical companies have Web sites listing
available clinical trials.
 Hundreds of cancer trials for all kinds of
treatments are taking place worldwide. For details,
see the National Cancer Institute Web site.
Hundreds of cancer trials for all kinds of
treatments are taking place worldwide. For details,
see the National Cancer Institute Web site.
|
|
The National Cancer Institute (NCI) has a comprehensive
database of information about cancer research studies. This
database includes information about specific types of
cancer, information about finding ongoing trials (including
a list of angiogenesis inhibitors in clinical trial), and a
list of NCI-designated Cancer Centers by geographic region.
Specific trials are listed in a database produced by the
National Cancer Institute called PDQ (physician data query).
In addition to specific trials, the PDQ describes the latest
advances in cancer screening, prevention, and treatment.
This information is available to patients and their families
as well as to health-care professionals. For more
information about these clinical trials, you may contact the
National Cancer Institute's Cancer Information Service at
1-800-4-CANCER (1-800-422-6237) or on the Internet at
http://www.cancer.gov/cancer_information/
or
http://cancertrials.nci.nih.gov.
Before you make a decision about enrolling in a clinical
trial, including a trial testing an angiogenesis inhibitor,
you may want to confer with your physician. He or she can
help you put information about clinical trials into
perspective and help you decide what is appropriate for you.
|
 Judah Folkman can only wait and watch as his team's
angiogenesis inhibitors go through clinical trials.
Judah Folkman can only wait and watch as his team's
angiogenesis inhibitors go through clinical trials.
|
How long will I be treated with an angiogenesis
inhibitor?
Since angiogenesis inhibitors are in the early stages of
testing, it is not known how long this type of drug will
have to be used to be most effective. Information about a
specific drug's effectiveness, toxicity, long-term side
effects, and the development of drug resistance or lack of,
will help determine how long the course of treatment with an
angiogenesis inhibitor will be. When you are part of a
clinical trial testing an angiogeneis inhibitor, you will be
expected to participate for a pre-defined minimum period of
time, depending on the phase of the trial. However, in most
clinical trials, if a patient is not experiencing any
unexpected toxicities, and the tumor is stable or shrinking,
the patient may continue on treatment.
Q8. Can I use an angiogenesis inhibitor with the
chemotherapy that I am on right now?
A: Angiogenesis inhibitors currently in clinical
trial are part of protocols written specifically to test
these drugs for safety and effectiveness. Until these drugs
are approved by the FDA, they must be tested in strictly
monitored studies. Only anticancer drugs that are part of
the investigational protocol may be used.
Q9. Are these drugs available for compassionate
use?
A: Permission for compassionate use of a drug is
given by the FDA and drug study sponsor. Until more is known
about a drug's safety and effectiveness, it is not available
for individual patients who are not part of a clinical
trial.
Q10. Are angiogenesis inhibitors in clinical
trial for children with cancer?
A: Angiogenesis inhibitors must be tested for safety
in adult cancer patients first. If a drug proves to be safe,
the FDA may allow it to be tested in a clinical trial for
children with cancer. At this time there are only a few
angiogenesis inhibitors in clinical trial for children and
these studies are limited in number and size. For
information about angiogenesis inhibitors in clinical trial
for children with cancer, you may consult your child's
oncologist or contact the National Cancer Institute's Cancer
Information Service at 1-800-4-CANCER (1-800-422-6237) or on
the internet at
http://cancernet.nci.nih.gov
or
http://cancertrials.nci.nih.gov.
 Robert D'Amato, a member of Judah Folkman's team,
discovered that thalidomide was a potent
angiogenesis inhibitor.
Robert D'Amato, a member of Judah Folkman's team,
discovered that thalidomide was a potent
angiogenesis inhibitor.
|
|
Q11. Can angiogenesis inhibitors be used for
other diseases?
A: The role of angiogenesis and its contribution to
the development of other diseases is being widely studied.
In the future, these diseases may be treated with
angiogenesis inhibitors.
Currently, angiogenesis inhibitors are in clinical trial for
certain eye diseases. For information about these clinical
trials for eye disease, you may consult your physician or
contact the Macular Degeneration Foundation at
1-888-633-3937 or
www.eyesight.org.
Q12. How can I find out about clinical trials
for endostatin?
A: EntreMed is conducting several Phase I safety
studies of endostatin. These trials are nearing completion
and limited openings are available. Enrollment in these
trials is restricted to patients who live in the same area
as the trial center. Patients seeking additional information
about these trials should call the University of Wisconsin
(Madison) Cancer Connect Line at 1-800-622-8922 or
608-262-5223, or MD Anderson Cancer Center (Houston, Texas)
Information line at 1-800-392-1611 (Select Option 3) or
713-792-6161.
Q13. When will Phase II endostatin trials
begin?
A: Plans for Phase II clinical trials for endostatin
are underway. These trials will help determine endostatin's
effectiveness. Data from the Phase I trials (for example,
results on routes of administration, schedules of
administration, and dosages) will be used to design the
Phase II trials. These Phase II trials are expected to begin
during the second quarter of this year. Phase II clinical
trial sites have not yet been selected, and there are no
"waiting lists" for patients at this time.
Q14. How can I find out about clinical trials
for angiostatin?
A: In the United States, there are two Phase I
clinical trials evaluating the safety of angiostatin in
humans. These trials are sponsored by EntreMed, Inc. and are
being conducted at Thomas Jefferson University Hospital in
Philadelphia, Pennsylvania. One of the studies is testing
angiostatin as a single agent, and the other study is
testing angiostatin in combination with radiation therapy.
Enrollment in these trials is limited to patients who live
in the same area as the trial center. Patients seeking
additional information about these trials may call
1-800-JEFF-NOW or contact the Thomas Jefferson University
Web site at
www.kcc.tju.edu.
|
 Dr. Folkman does research full-time and no longer
sees patients.
Dr. Folkman does research full-time and no longer
sees patients.
|
Q15. Does Dr. Folkman see patients?
A: Dr. Folkman is a physician, Professor of Surgery
and Cell Biology at Harvard Medical School, and Director of
the Surgical Research Laboratory at Children's Hospital in
Boston. He is devoting his full effort to scientific
research and does not see patients.
There are many excellent oncologists who are treating
patients throughout the United States and worldwide, and
most cancer centers have a referral service for patients
seeking an oncologist. For a list of NCI-designated cancer
centers by geographic region, you may contact the National
Cancer Institute's Cancer Information Service at
1-800-4-CANCER (1-800-422-6237) or on the Internet at
http://cancernet.nci.nih.gov.
Dr. Folkman Speaks
|
Cancer Caught on Video
Designing Clinical Trials
|
Accidental Discoveries
| How Cancer Grows
Help/Resources
|
Transcript
|
Site Map
|
Cancer Warrior Home
Editor's Picks
|
Previous Sites
|
Join Us/E-mail
|
TV/Web Schedule
About NOVA |
Teachers |
Site Map
|
Shop |
Jobs |
Search |
To print
PBS Online |
NOVA Online |
WGBH
©
| Updated February 2001
|
|
|