Diamond Factory
Visit a laboratory where entrepreneurs are growing perfectly pure diamonds. Airing June 30, 2009 at 9 pm on PBS Aired June 30, 2009 on PBS
Program Description
Transcript
Diamond Factory
PBS Airdate: June 30, 2009
NEIL DeGRASSE TYSON (As Treasure-Hunter Indiana Jones): Treasure hunters are known for taking risks, especially if they're after something as valuable as a giant diamond.
These days, big diamonds are in demand, and not just for jewelry. Some think they hold a key to a new age of electronics. ...problem is the right kinds of diamonds are extremely rare in nature and hard to find.
(As Scientist Indiana Jones): Dr. Tyson, we have an answer.
(As Treasure Hunter Indiana Jones): Some scientists have a new recipe for diamonds, made, not in the earth, but in the lab.
Recently, I got to visit a secret place. I can't tell you where it is, because, well, I don't really know where it is. I could only get there if I agreed to be blindfolded.
BRUCE LIKELY: It will be a little while driving, so make yourself comfortable.
NEIL DeGRASSE TYSON: In fact, the location is so secret, our entire film crew had to be blindfolded.
When I finally got to peek, it didn't look all that unusual.
Where am I? Oh, sorry!
But don't let appearances fool you.
ROBERT LINARES (Apollo Diamond, Inc.): You're at the U.S. diamond factory.
NEIL DeGRASSE TYSON: A diamond factory?
Or maybe a better term would be a diamond "farm," because, as I discovered, what they're doing in the back rooms of this ordinary office building is actually growing diamonds.
BRYANT LINARES: The diamonds we grow are 100 percent real diamond. They are chemically, physically and optically identical to mined diamonds, with one exception: we grow them.
NEIL DeGRASSE TYSON: And some of the diamonds they grow here are beautiful.
So basically, you're making diamonds in machines like this?
But Robert Linares didn't start this company simply to make bling. He wants to make diamonds that will revolutionize technology.
ROBERT LINARES: Diamond is going to have a huge worldwide impact for the next 50 years.
BRYANT LINARES: It's going to be in our cell phones. It's going to be in our electric cars and in our power grid. It will be everywhere.
NEIL DeGRASSE TYSON: The company hopes to realize the potential that scientists have seen in diamond for years. Because it's not just pretty, it's one of the most impressive materials in the universe.
PATRICK DOERING (Apollo Diamond, Inc.): Diamond has an amazing toolkit of properties.
JAMES E. BUTLER (Naval Research Laboratory): You can boil it in any acid or any base and it doesn't destroy diamond.
PATRICK DOERING: The highest velocity of sound is in diamond. If you could speak to somebody through diamond, the sound would get there much faster than it would through air.
NEIL DeGRASSE TYSON: And it's not just sound that travels quickly through a diamond. Heat moves faster through a diamond than any other known substance.
Jim Butler at the Naval Research Lab demonstrated this to me with a simple experiment...
JIM BUTLER: Do you have a credit card?
NEIL DeGRASSE TYSON: ...and a block of ice.
I only just met you, so how about my triple-A card?
JIM BUTLER: That's fine with me.
Press against the block and count the number of seconds until your fingers get cold.
NEIL DeGRASSE TYSON: This is going to take awhile.
JIM BUTLER: This is going to take awhile, so it's not going to happen. And did it make any dent in the ice?
NEIL DeGRASSE TYSON: Okay, I don't see any. Nothing, there's nothing there.
JIM BUTLER: So, how about a piece of copper? So hold it by the edge.
NEIL DeGRASSE TYSON: This'd be like holding a penny.
JIM BUTLER: Yeah, holding a penny, but this is just a nice, pure piece of copper.
NEIL DeGRASSE TYSON: About a half inch from the edge.
JIM BUTLER: Okay, now count the number of seconds.
NEIL DeGRASSE TYSON: Oh, right there!
JIM BUTLER: So, how many seconds?
NEIL DeGRASSE TYSON: That was three seconds.
JIM BUTLER: Three to four seconds. And what did you do to the ice cube?
NEIL DeGRASSE TYSON: It's still cold. I cut the ice.
This small piece of copper sliced right through the ice, because, unlike the plastic card, copper is an excellent conductor of heat. So it drew the heat right out of my fingertips, melting the ice and leaving my fingers cold. Impressive, huh?
But then, Jim offered me a big chunk of diamond.
JIM BUTLER: Hold it just by the tip. Now, touch the ice and count the seconds.
NEIL DeGRASSE TYSON: Instant. It's cutting through the ice like a knife through butter.
JIM BUTLER: Yes.
NEIL DeGRASSE TYSON: It turns out diamond conducts heat five times faster than copper.
All this is possible because of diamond's unusual crystal structure. Pure diamond is made of all carbon atoms, but the carbon atoms have to be arranged in a unique way to give it the amazing properties of diamond.
If you arrange the carbon atoms in another way, you get this: graphite, one of the softest materials around.
STEPHEN STEINER: Well, obviously, these are two very different materials. Graphite is black, it's opaque; when we rub it against paper, it comes apart. Whereas diamond is transparent; it's hard, one of the hardest substances known to mankind.
PATRICK DOERING: Diamond has the highest atomic density of any material. So there's more atoms per cubic centimeter than any other material.
NEIL DeGRASSE TYSON: But diamond isn't just hard, it has impressive electrical properties, as well.
STEPHEN STEINER: A centimeter-thick plate of diamond can withstand 10 million volts of electricity.
NEIL DeGRASSE TYSON: Electrical engineers would love to exploit these unique properties, but they've been frustrated by the ones that come out of the ground, because they're all slightly different.
JIM BUTLER: The reason we cannot use diamonds out of the ground for a lot of technological applications is because no two are alike. They're like snowflakes. Have you ever seen two snowflakes that are alike? We've got billions of diamonds that we've mined out of the ground but trying to find two that have exactly the same properties is actually very difficult.
NEIL DeGRASSE TYSON: It all comes down to how natural diamonds form. Most natural diamonds formed billions of years ago, deep beneath Earth's crust, under extreme pressures and temperatures. Volcanic activity transported them to the surface, where we find them today.
PATRICK DOERING: So the earth is not a really well-controlled crystal growth furnace. So what happens is you're left with whatever the earth gives you. You have to then scratch your head and say, "What can I do with it?"
NEIL DeGRASSE TYSON: Decades ago, engineers figured out how to make diamonds in giant, hot vises. But the process is impractical for making large stones, and it's difficult to produce a crystal that's pure carbon without defects.
But what if you can manufacture diamonds and guarantee purity and consistency?
ROBERT LINARES: Neil, you have to put your glasses on.
NEIL DeGRASSE TYSON: The folks at Apollo Diamond say they can do it.
They're one of just a few companies making diamonds using technology known as "chemical vapor deposition," or C.V.D.
PATRICK DOERING: So, to grow a diamond, you have to start with a piece of diamond. So you start with a very thin plate of diamond. It's as thick as a human hair.
NEIL DeGRASSE TYSON: Here's how it works: they start with thin slices of pure diamond called "seeds." The seeds are then placed inside a vacuum chamber; a cocktail of gasses is pumped in. Apollo's exact recipe is top-secret, but it, of course, includes gasses that contain carbon—such as methane—which are heated to extreme temperatures, so they become what's called a "plasma."
So what temperature is that plasma?
PATRICK DOERING: That plasma—if you could measure—the temperature's probably 3,000 or 4,000 degrees Celsius. It's about the same temperature of the gas that you'd find at the edge of the sun.
NEIL DeGRASSE TYSON: The extreme heat breaks apart the gas molecules, and then, through a complex chain of reactions, carbon atoms take their place on the crystal below, following the pattern established by the diamond seed.
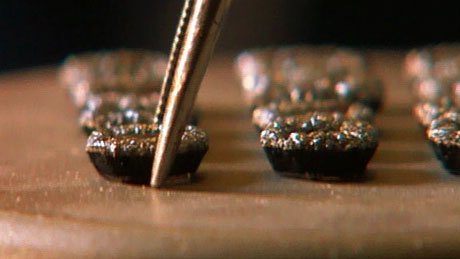
Over the course of a week or more, diamonds grow bigger and bigger. When they emerge from the grower, they don't look much like diamonds, until they're cut and polished. And then...
The diamonds baked up here look great to me, but what would an expert think? To find out, I took a sample to the famous diamond district in New York City, where I met Ara Arslanian, who buys and cuts some of the most impressive stones in the world.
ARA ARSLANIAN: Fifty carat, they will charge you four million.
NEIL DeGRASSE TYSON: Aah! Not 10,000, not 100,000?
ARA ARSLANIAN: No, no, no. This is real, the real McCoy.
NEIL DeGRASSE TYSON: This is the real thing?
ARA ARSLANIAN: This is the real thing.
NEIL DeGRASSE TYSON: So, you know why I'm here.
So, even though small stones aren't usually his thing, Ara agreed to participate in our little experiment.
Point-two-nine and point-three carat.
I showed Ara three small cut gemstones. One was grown in Apollo's lab, the two others came from diamond mines.
ARA ARSLANIAN: ...so tiny. I'm not used to those.
NEIL DeGRASSE TYSON: As I told you, at least one of these is manmade.
ARA ARSLANIAN: Let me put this thing on my telescope. Here we go.
NEIL DeGRASSE TYSON: I see them sparkle from here...
ARA ARSLANIAN: Yeah.
NEIL DeGRASSE TYSON: ...as all good diamonds should do.
ARA ARSLANIAN: They pretty much look the same. So, normally, I'm a rough guy; I do the rough diamonds. If I have to guess—well it's a wild guess—I would guess this one.
NEIL DeGRASSE TYSON: That's the .3 carat.
The one that is manmade is this one.
ARA ARSLANIAN: But I know what I'm seeing, I'm seeing here. I couldn't distinguish which one is manmade and the natural.
NEIL DeGRASSE TYSON: With the customary jeweler's loupe, Ara couldn't detect any difference in the manmade diamond.
ARA ARSLANIAN: Yeah, it's amazing what they can do. It's like a toupee. It's the feeling which got...you can...which one you prefer, the toupee or your real hair?
NEIL DeGRASSE TYSON: The real hair.
ARA ARSLANIAN: Even if the toupee looks great.
NEIL DeGRASSE TYSON: More advanced equipment can reveal subtle differences, and Apollo isn't trying to trick anyone. In fact, their manmade gemstones are marked with a microscopic brand, so the people who buy them know just what they're getting.
But for scientists, what's most exciting about growing diamonds is not how you can make them just like natural stones but how you can make them different.
Because with chemical vapor deposition, or C.V.D., diamonds can be grown into shapes and sizes nature could never produce.
And you can tinker with the recipe.
JIM BUTLER: In a C.V.D. diamond we can actually control how we grow the diamonds. We can engineer the material to have the property match the application that we need.
NEIL DeGRASSE TYSON: For example, add a little bit of the element boron to the carbon gas mixture, and you get a blue diamond. That's where the famous Hope Diamond gets its distinctive color, but boron does more.
PATRICK DOERING: So when you put the boron in it, it not only makes the diamond blue, it also changes its electrical properties. So a diamond with no boron, perfectly pure
diamond, basically, you can't get any electricity to flow through it. When you put boron in, you can now get electricity to flow through the diamond. And this is an essential component, if you want to make an electronic device.
NEIL DeGRASSE TYSON: Today, most electronic devices, from computer chips to televisions, are built from silicon. But silicon has its limits.
STEPHEN STEINER: Silicon is so 20th century. It's time to move on. Silicon has some fundamental drawbacks: it fails when it gets hot. Somewhere around the boiling point of water, it starts to break down. It's not able to process information anymore.
Well, it turns out that there are a number of features of diamond which blow silicon out of the water.
NEIL DeGRASSE TYSON: So, take an electric train. Today, these modern machines carry tons of silicon transistors to manage the high-voltage electricity coming into the train. But what if you could make those transistors out of diamond instead?
STEPHEN STEINER: Diamond can come to the rescue. Diamond has the ability to switch much higher frequencies, much higher voltages.
JIM BUTLER: Then all of that electronics could be simplified, the weight could be removed. You could envision that your train, instead of having one to two tons of electronics per railcar, might have only fifty pounds of electronics per railcar.
NEIL DeGRASSE TYSON: And a lighter train is more energy efficient.
The field of diamond electronics is in its infancy, and a lot more work needs to be done before diamond starts replacing silicon, but the potential is there, and the diamond growers have big dreams: diamond switches that could improve our aging electrical grid, diamond windows for spacecraft, and who knows what else?
STEPHEN STEINER: Mother Nature was the only manufacturer of diamond for a really long time. And that's what's so exciting about a material like diamond...is because it's kind of been kept in a little treasure box for a long time, and now, we've just begun to open that treasure box, and all of the possibilities which come with it are beginning to emerge, as well.
On Screen Text: So what is Apollo's secret ingredient? They're still not saying. But diamond-making scientists at the University of Nuevo Leon, Mexico use tequila. We're not kidding. They created diamonds using a similar process and their own "secret ingredient."
Broadcast Credits
Diamond Factory
- Edited by
- Steve Audette
- Written, Produced and Directed by
- Julia Cort
NOVA scienceNOW
- Executive Producer
- Samuel Fine
- Executive Editor
- Neil deGrasse Tyson
- Senior Series Producer
- Vincent Liota
- Senior Producer
- Julia Cort
- Supervising Producers
- Stephen Sweigart
Joey David Jovanovich - Senior Editor and Colorist
- David Chmura
- Senior Researcher
- Sharon Kay
- Associate Producer
- Fran Laks
- Assistant Editor
- Tung-Jen (Sunny) Chiang
- Graphic Design
- Brian Edgerton
- Compositor & Animator
- Yunsik Noh
- Music
- Rob Morsberger
- Sound Mix
- Bill Cavanaugh, RazorMix, Inc.
- Assistant to Neil deGrasse Tyson
- Elizabeth Stachow
- NOVA scienceNOW Series Animation
- Edgeworx
- Associate Producers
- Julie Crawford
Jonathan Loewald
Richard Marnell
Laura Willcox - Camera
- Austin de Besche
Brian Dowley
Robin Hirsh
Stephen Kazmierski
Rob Lyall - Sound Recordists
- John Cameron
James Lindsey
Len Schmitz
Chris Strollo - Animation and Graphics
- Anthony Kraus
Sputnik Animation
James LaPlante
Dan Nutu - Researchers
- Ethan Herberman
Eric Olson - Archival Material
- AP Worldwide Photos
BBC Motion Gallery
CNN Image Source
Corbis
The Frederick News-Post and Randall Family, LLC
Getty Images
Josh Landis
National Science Foundation
NASA
Prelinger Internet Archives
The San Diego Union-Tribune
Schenectady Museum & Suits-Bueche Planetarium
Steven Snyder
WTC: The First 24 Hours, Producers; Etienne Sauret & David Carrara - Special Thanks
- Jason Bannan, FBI microbiologist
Carnegie Mellon University
Dave and Busters
Gemological Institute of America
Justin Goger Malo
George Gray, Gray Jewelers
Susan Hrishenko
Northern Arizona University
David Rasko
Sandia National Laboratories
University of Maryland School of Medicine - Neil deGrasse Tyson
- is director of the Hayden Planetarium in the Rose Center for Earth and Space at the American Museum of Natural History.
- NOVA Series Graphics
- yU + co.
- NOVA Theme Music
- Walter Werzowa
John Luker
Musikvergnuegen, Inc. - Additional NOVA Theme Music
- Ray Loring
Rob Morsberger - Post Production Online Editor
- Spencer Gentry
- Closed Captioning
- The Caption Center
- NOVA Administrator
- Mykim Dang
- Publicity
- Carole McFall
Eileen Campion
Victoria Louie
Karinna Sjo-Gaber
Karen Laverty - Marketing
- Steve Sears
- Researcher
- Kate Becker
- Senior Researcher
- Gaia Remerowski
- Production Coordinator
- Linda Callahan
- Paralegal
- Sarah Erlandson
- Talent Relations
- Scott Kardel, Esq.
Janice Flood - Legal Counsel
- Susan Rosen
- Production Assistant
- Ryan Murdock
- Post Production Assistant
- Darcy Forlenza
- Associate Producer, Post Production
- Patrick Carey
- Post Production Supervisor
- Regina O'Toole
- Post Production Editors
- Rebecca Nieto
Jason York - Post Production Manager
- Nathan Gunner
- Compliance Manager
- Linzy Emery
- Development Producer
- Pamela Rosenstein
- Business Manager
- Joseph P. Tracy
- Senior Producer and Project Director
- Lisa Mirowitz
- Coordinating Producer
- Laurie Cahalane
- Senior Science Editor
- Evan Hadingham
- Senior Series Producer
- Melanie Wallace
- Managing Director
- Alan Ritsko
- Senior Executive Producer
- Paula S. Apsell
This material is based upon work supported by the National Science Foundation under Grant No. 0638931. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
NOVA scienceNOW is a trademark of the WGBH Educational Foundation
NOVA scienceNOW is produced for WGBH/Boston by NOVA
© 2009 WGBH Educational Foundation
All rights reserved
- Image credit: (synthetic diamonds) © WGBH Educational Foundation
Participants
- Ara Arslanian
- Cora International www.corainternational.com/about.php
- James Butler
- Naval Research Laboratory
- Neil deGrasse Tyson
- Astrophysicist, American Museum of Natural History www.haydenplanetarium.org/tyson/profile/bio
- Patrick Doering
- Apollo Diamond www.apollodiamond.com/
- Bryant Linares
- Apollo Diamond www.apollodiamond.com/
- Robert Linares
- Apollo Diamond www.apollodiamond.com/
- Stephen Steiner
- Massachusetts Institute of Technology
Preview
Full Program | 14:35
Full program available for streaming through
Watch Online
Full program available
Soon