
|

|
|
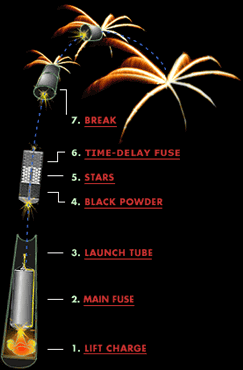
Anatomy of a Firework
Click on labels for details
 When most of us think "fireworks," we think of brilliant
bursts of light and color we've seen paint a night sky. But
such bursts are merely the spectacular end of
fireworks that likely took centuries of experience, weeks of
planning, and hours of painstaking labor to fashion and
fire. In this feature, pull back the wrapping on a typical
aerial display shell and see what it looks like
before its glorious denouement in the dark.
When most of us think "fireworks," we think of brilliant
bursts of light and color we've seen paint a night sky. But
such bursts are merely the spectacular end of
fireworks that likely took centuries of experience, weeks of
planning, and hours of painstaking labor to fashion and
fire. In this feature, pull back the wrapping on a typical
aerial display shell and see what it looks like
before its glorious denouement in the dark.
Click on the labels at left to learn more about them.
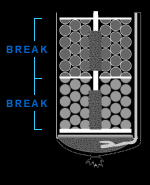 Break
Break
In a multi-break firework, stars are contained in separate
cardboard compartments within the shell. Each compartment
has its own bursting charge, which ignites and throws out
the stars. In order to spread these decorations over a wide
area of sky, the break must burst open with tremendous
force. The more the compartment can resist the explosion and
bottle up its force, the bigger the display will be.
Resistance comes from the break's heavy wrapping, which
momentarily keeps the gas and heat from reaching the
bursting charge.
A firework's breaks may also contain sound charges, which
result in the cracking bangs and thunderous booms that
thrill audiences. To make these loud explosions, which are
usually accompanied by a bright white flash, firework
manufacturers use mixtures of perchlorate, a different kind
of explosive than black powder.
Back to main diagram
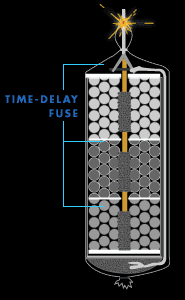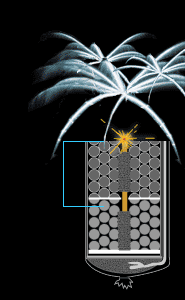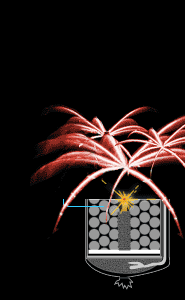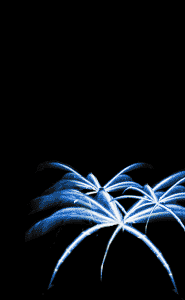 Time-delay fuse
Time-delay fuse
As the firework ascends through the air, the time-delay fuse
continues to burn. By the time the shell nears its apogee,
the fuse has burned low enough to ignite the black powder in
the first break (or compartment). Colored stars ignite in
every direction. But the show isn't over yet. The fuse keeps
burning, making its way toward the stashes of black powder
in the second and third breaks.
Timing is critical. In a three-break firework, the middle
break needs to ignite at the highest point in the shell's
trajectory. Thus, the first break should blow a little
before and the third break a little after. If the timing is
off, the firework might detonate too close to the ground.
Great care is used in designing the fuses and calculating
their lengths.
Back to main diagram
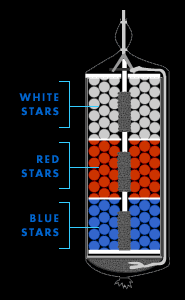 Stars
Stars
Stars are the precious cargo carried by "aerial" fireworks
like the one depicted here. An unlit star isn't much to look
at—just a dull black lump about the size of a
jawbreaker. But appearances can be deceiving. When ignited,
stars create the breathtaking flashes of color and light
that elicit "oohs"and "ahhhs" from even the most jaded
spectators.
Fireworks masters manufacture their creations by hand,
including the hundreds of stars that go into a single
firework. They mix carefully measured ingredients like
perchlorate and black powder with binding and coloring
agents: magnesium or aluminum for white, sodium salts for
yellow, strontium nitrate or carbonate for red, barium
nitrate for green, copper salts for blue, and charcoal or
other forms of carbon for orange. The result is a huge slab
of dough, which is then cut like a tray of brownies into
half-inch cubes; these are then set out to dry.
Stars can be extremely dangerous if not handled and stored
with care. A sharp blow can detonate one. Oil from nearby
machines can combine with certain chemicals to create an
explosive gas. Even synthetic clothing, which generates
static electricity, can create sparks capable of detonating
the fragile shells. Firework makers must stick to wearing
cotton—all the way down to their underwear.
Back to main diagram
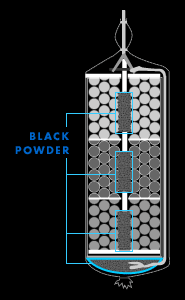 Black powder
Black powder
The recipe for black powder, or gunpowder, the basic
material in all fireworks, has remained the same since it
was discovered in China about 1,000 years ago: 75 percent
saltpeter (potassium nitrate), 15 percent charcoal, and 10
percent sulfur. Black powder lends itself to fireworks
because it's a "low explosive," meaning its detonation
velocity is less than about 100 yards per second. ("High
explosives" like dynamite have a velocity of detonation
greater than 1,000 yards per second.) Fireworks makers can
also control the powder's rate of burn in several ways. One
way is by manipulating the size of its grains: Fine grains
burn more quickly than coarse grains.
Back to main diagram
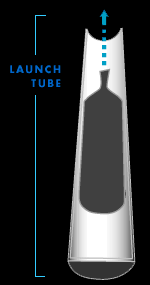 Launch tube
Launch tube
Most fireworks are launched from rows of steel tubes secured
in troughs of sand. The tubes, or "mortars," are three times
as long as the firework shells but have the same diameter.
If a firework doesn't fit snugly into its launch tube, the
pressure created by the lift charge will escape, and the
firework can misfire.
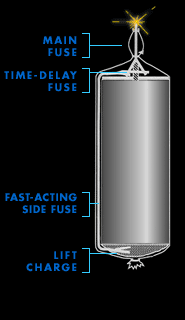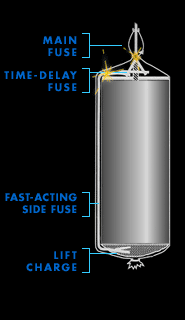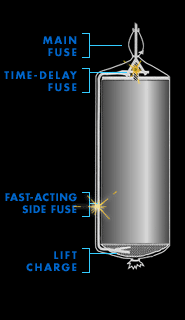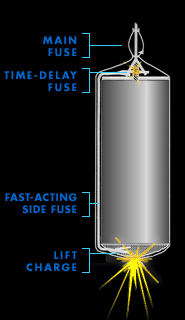 Main fuse
Main fuse
During the Renaissance, when fireworks as we know them were
invented, pyrotechnicians lit their creations with tissue
paper rolled around a trail of black powder. Later, string
embedded with gunpowder was used. Today, electrical wires
connect fireworks to a master control board. With the push
of a button, an electrical current rushes through each wire
and creates a spark at the point of contact on the main
fuse.
The main fuse simultaneously lights two secondary
fuses—a fast-acting side fuse that ignites the lift
charge, and a time-delay fuse buried inside the shell that
leads to the heart of the firework.
Back to main diagram
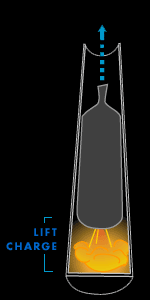 Lift charge
Lift charge
When black powder burns in the open air, the heat and gas it
generates quickly dissipate. But if the black powder is
confined, say in a pouch at the bottom of a firework
cylinder, the trapped heat and gas will push vigorously at
the inside of the launch tube until an explosion results.
This explosion will free the heat and gas and hurtle the
firework shell as high as 1,000 feet into the air.
Back to main diagram
Name That Shell
|
Anatomy of a Firework
|
Pyrotechnically Speaking
|
On Fire
Resources
|
Transcript
|
Teacher's Guide
|
Site Map
|
Fireworks! Home
Search |
Site Map
|
Previously Featured
|
Schedule
|
Feedback |
Teachers |
Shop
Join Us/E-Mail
| About NOVA |
Editor's Picks
|
Watch NOVAs online
|
To print
PBS Online |
NOVA Online |
WGBH
©
| Updated January 2002
|
|
|
|