

|
|
|

|
On Fire Step Through the Reaction Back to Chain Reaction 
Step 1 The chemical reactions of hydrogen combustion occur because certain atoms and molecules are very reactive—they are unstable on their own and quickly combine with other molecules or atoms. Adding the single hydrogen atom to the mixture of oxygen and hydrogen gas, as you did, led to a series of chemical reactions. 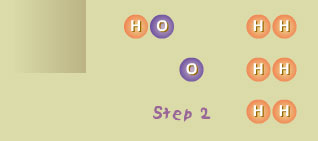
Step 2 The single hydrogen atom (H) reacted with a molecule of oxygen (O2), producing a molecule of hydroxyl radical (OH) and an atom of oxygen (O). Both OH and O are also highly reactive. 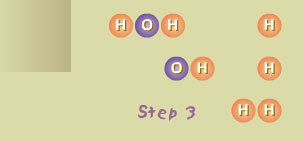
Step 3 The OH molecule reacted (very rapidly) with a hydrogen molecule (H2), producing a water molecule (H2O) and another hydrogen atom (H). The oxygen atom liberated in Step 2 reacted with H2, producing new OH and H. 
Step 4 The new OH molecule reacted with another H2 molecule, producing another molecule of water (H2O) and yet another hydrogen atom (H). 
Steps 5 - The three hydrogen atoms liberated by this reaction quickly reacted with three other O2 molecules, which resulted in the liberation of nine other hydrogen atoms. Those nine liberated 27 more, then those 27 liberated 81, and so on. This is known as a branching chain reaction. Next: Shuffle Atoms. Strike a Match | Shuffle Atoms | Flame Experiment
The Producer's Story | The World on Fire | Outfitting Wildland Firefighters How Plants Use Fire | Glossary of Fire Terms | Wildfire Simulator | On Fire Resources | Transcript | Site Map | Fire Wars Home Search | Site Map | Previously Featured | Schedule | Feedback | Teachers | Shop Join Us/E-Mail | About NOVA | Editor's Picks | Watch NOVAs Online | To Print PBS Online | NOVA Online | WGBH © | Updated June 2002 |