

|
|
|

|
On Fire Shuffle Atoms (2) 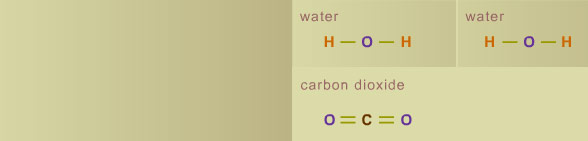
The illustration above shows a methane molecule that has oxidized completely. Sometimes the combustion of a fuel is not complete. For example, if the fuel mixture is too rich—that is, the fuel-to-oxygen ratio is too high—there will not be enough oxygen to allow the fuel to fully oxidize. This can result both in the formation of carbon monoxide (CO) instead of CO2 and in a sooty flame. Next, find out what make fire visible with a Flame Experiment. Strike a Match | Chain Reaction | Flame Experiment
The Producer's Story | The World on Fire | Outfitting Wildland Firefighters How Plants Use Fire | Glossary of Fire Terms | Wildfire Simulator | On Fire Resources | Transcript | Site Map | Fire Wars Home Search | Site Map | Previously Featured | Schedule | Feedback | Teachers | Shop Join Us/E-Mail | About NOVA | Editor's Picks | Watch NOVAs Online | To Print PBS Online | NOVA Online | WGBH © | Updated June 2002 |