

|
|
|

|
On Fire Flame Experiment (2) 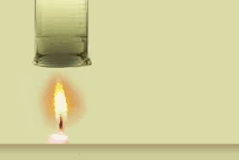
Notice the black soot underneath the beaker. This soot, which is made up mainly of carbon, formed just before it entered the flame. Within the flame of the candle, the carbon atoms don't completely combine with the oxygen. Instead, they combine with other surrounding atoms to form large molecules consisting mainly of carbon. These soot molecules get so hot that they glow. The orange-red flame color comes from the glowing soot as it feeds into the flame. That's it for On Fire. If you have skipped any of the four sections, select that section below. Strike a Match | Chain Reaction | Shuffle Atoms
The Producer's Story | The World on Fire | Outfitting Wildland Firefighters How Plants Use Fire | Glossary of Fire Terms | Wildfire Simulator | On Fire Resources | Transcript | Site Map | Fire Wars Home Search | Site Map | Previously Featured | Schedule | Feedback | Teachers | Shop Join Us/E-Mail | About NOVA | Editor's Picks | Watch NOVAs Online | To Print PBS Online | NOVA Online | WGBH © | Updated June 2002 |