
|
 |
|
 The Atom Builder Guide to Building a Stable Atom
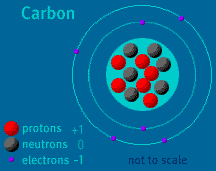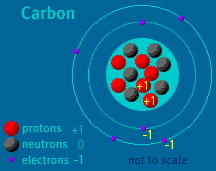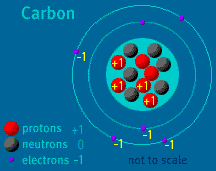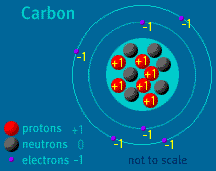
The Atom Builder Guide to Building a Stable AtomBack to See Inside a Diamond Back to Atom Builder Shockwave When building an atom, pay close attention to the particles' electrical charges. Protons have a charge of +1. Electrons have a charge of -1. Neutrons are neutral, as their name implies. A stable atom has a net charge of 0. In other words, it has an equal number of protons and electrons. The positive protons cancel out the negative electrons. When the number of electrons does not equal the number of protons, the atom is ionized. (The atom is then called an ion). Do not allow your atom to become too ionized. Ions are attracted to other atoms and molecules. If your atom is too ionized, it will likely zip away from you and attach itself to a nearby atom or molecule. An atom becomes radioactive when its nucleus contains too many or too few neutrons. Try to keep the same number of neutrons and protons as you build your atom. If the imbalance is too great, radioactive decay will occur. Carbon-14, to take one example, is unstable. Its nucleus contains six protons and eight neutrons. When carbon-14 decays, one of its neutrons changes into a proton and an electron is released. The atom, which then contains seven protons and seven neutrons, is no longer carbon—it is Nitrogen-14. Up to two electrons may exist in the atom's first shell. Up to eight electrons may exist in the atom's second shell. An electron may "drop" from an outer shell if an inner shell is not full. This results in the release of energy in the form of a photon. Atomic Discovery: A Brief History The Atom Builder Guide to Elementary Particles Back to the first page of See Inside a Diamond The Science Behind the Sparkle | Diamonds in the Sky A Primer of Gemstones | See Inside a Diamond Resources | Transcript | Site Map | Diamond Deception Home Editor's Picks | Previous Sites | Join Us/E-mail | TV/Web Schedule About NOVA | Teachers | Site Map | Shop | Jobs | Search | To print PBS Online | NOVA Online | WGBH © | Updated November 2000 |