

|

|
|
|
|
|
Discovering Air A version of this article first appeared on NOVA's Web site Everest.  Before the age of science, it was clear to most observers that air
was a "vital spirit," but beyond that, little was known about the
physical properties of air. Aristotle suggested that air was one of
four elements that also included earth, water, and fire, and for
2000 years this was the prevailing view. But beginning in the 17th
century, a new era of scientific inquiry and discovery began to
reveal the true nature and behavior of the air we breathe.
Before the age of science, it was clear to most observers that air
was a "vital spirit," but beyond that, little was known about the
physical properties of air. Aristotle suggested that air was one of
four elements that also included earth, water, and fire, and for
2000 years this was the prevailing view. But beginning in the 17th
century, a new era of scientific inquiry and discovery began to
reveal the true nature and behavior of the air we breathe.
1618: Issac Beekman compares air to water, and the pressure water exerts at depth, and comes up with the then radical suggestion that air has physical properties. Even Galileo and Descartes steadfastly disagree. 1640: Gaspar Berti, through an inventive experiment with a lead tube and bucket of water, demonstrates how an air vacuum can be created.  1643: Evangelista Torricelli conducts Berti's experiment with
mercury instead of water. This leads to the invention of the
barometer, which measures atmospheric pressure (a measure of the
pressure exerted by the atmosphere on a surface).
1643: Evangelista Torricelli conducts Berti's experiment with
mercury instead of water. This leads to the invention of the
barometer, which measures atmospheric pressure (a measure of the
pressure exerted by the atmosphere on a surface).1646: Pierre Petit tests Blaise Pascal's theory that atmospheric pressure decreases with height, by carrying a barometer to the top of a small mountain in France. This experiment confirms that the atmosphere has weight. 1660: Robert Boyle is perhaps the first to predict weather using the barometer. Boyle suggests that the process of combustion and respiration are due to a "nitrous" substance in air. He also creates "Boyle's Law," which says 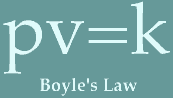 that the volume of air decreases as pressure on air increases (later
applied to all gases).
that the volume of air decreases as pressure on air increases (later
applied to all gases). 1671: Robert Hooke creates the first decompression chamber. He climbs inside a barrel, and has one tenth of the air sucked out, thereby simulating conditions at 3,000 feet; Hooke notices only a slight pressure on the ears. 1674: About this time, Johann Baptista van Helmont first uses the word "gas" to define a state of matter other than liquids or solids. John Mayow determines that air is composed of at least two gases -- one, which supports life and combustion, and another which does not. 1723: George Stahl claims, wrongly, that all substances are composed of water and three different kinds of earth -- one of them combustible -- which he termed phlogiston. "Phlogiston theory" prevails well into the 18th century. 1727: Stephen Hales proves that "air" can be released from solids and liquids. Other scientists soon begin trying to determine the properties of this "air." 1770: Carl Scheele is the first to create oxygen (although he does not understand what it is) from saltpeter, and is the first to challenge the popular phlogiston theory. In 1781 he publishes his book on gases. 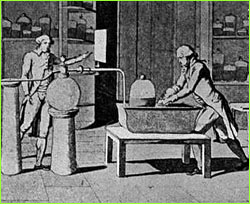 1772: Antoine Lavoisier and Joseph Priestley conduct
experiments similar to Scheele's in a new study of gases. Over the
next decade they work, independently of each other, to identify many
new "airs."
1772: Antoine Lavoisier and Joseph Priestley conduct
experiments similar to Scheele's in a new study of gases. Over the
next decade they work, independently of each other, to identify many
new "airs." 1778: Lavoisier first describes his oxygen theory of combustion -- whereby oxygen in air is the necessary component for combustion -- not phlogiston in solids. It would be many more years before oxygen's role in respiration would be clearly understood.  1785: Based on his years of study, Lavoisier begins attacking
the phlogiston theory. While some (such as Priestley) stand by the
phlogiston theory, by the late 1790s, most have converted. Lavoisier
continues work on gases and identifying elements which Dmitri
Mendeleev, in 1869, turns into the Periodic Table of Elements. 200
years later, chemistry is still based on the foundation developed by
Lavoisier.
1785: Based on his years of study, Lavoisier begins attacking
the phlogiston theory. While some (such as Priestley) stand by the
phlogiston theory, by the late 1790s, most have converted. Lavoisier
continues work on gases and identifying elements which Dmitri
Mendeleev, in 1869, turns into the Periodic Table of Elements. 200
years later, chemistry is still based on the foundation developed by
Lavoisier.Climb | Expedition | Mountain of Extremes Denali for Kids | Dispatches | E-Mail | Resources Site Map | Surviving Denali Home Editor's Picks | Previous Sites | Join Us/E-mail | TV/Web Schedule About NOVA | Teachers | Site Map | Shop | Jobs | Search | To print PBS Online | NOVA Online | WGBH © | Updated November 2000 |
|