Covid-19 patients sharing ventilators is possible—but not ideal
The science of coventilation for coronavirus cases illustrates a complicated dilemma.

Less-invasive ventilators use a mask rather than a tube inserted into the patient's throat. Image Credit: Juanmonino, iStock
On March 20, as severe cases of COVID-19 spiked in northern Italy, emergency medicine doctor Marco Garrone paused during a chaotic shift to tweet a photo: two patients, next to each other in hospital beds, with arcs of tubing connecting them to the same ventilator. “This is what we are down to,” he wrote. “Splitting ventilators, and facing serious dilemmas like choosing who will be actually ventilated when everybody should. #TakeThisSeriously”
A month later, as caseloads skyrocketed across the pond in New York City, Columbia Presbyterian Hospital hurried to draft protocols for ventilator sharing. And around the same time, an emergency medicine doctor in Michigan named Charlene Babcock posted a YouTube tutorial featuring step-by-step directions on how to modify a ventilator so it can accommodate multiple patients. That video racked up nearly a million views in the ensuing weeks.
“Here’s my disclaimer,” Babcock says to the camera. “This is off-label use of the ventilator.” But, she adds, extreme circumstances may call for measures that otherwise would be deemed too risky. “If it was me, and I had four patients—and they all needed intubation, and I only had one ventilator—I would simply have a shared discussion with all four families and say: ‘I can pick one to live, or we can try to have all four live.’”
The appearance of ventilator sharing (or “coventilating”) this spring in places where the novel coronavirus has hit the most severely prompts a number of questions: How does a ventilator work? Why is it possible for more than one patient to use a ventilator at once? And if it’s possible, why aren’t more doctors in hard-hit areas doing it? Good news: This is the first in a NOVA series answering burning coronavirus questions just like these.
Have a COVID-related question for us? Follow the #CovidQs hashtag on Facebook and Twitter to submit your inquiry or ask us on Reddit @novapbs.
How do ventilators work?
Treating a patient in extreme respiratory distress is “like staring out the window and seeing people free fall,” says Albert Kwon, an anesthesiologist at New York Medical College. Doctors don’t know how long their patients have been “falling” or how long they’ll continue to fall without intervention; they must make an on-the-spot assessment about whether a parachute is necessary.
In that case, they can choose from several options, ranging from less to more invasive. All ventilators provide oxygen and promote its absorption in the bloodstream while also helping rid the body of the resulting carbon dioxide. The ones you’ve probably heard the most about during the COVID-19 pandemic provide a stream of air into the lungs via a tube inserted into a patient’s throat.
This stream of air exerts positive pressure, which is the opposite of how breathing usually works. When we breathe in on our own, our diaphragm muscles move down in our chests, increasing available space and creating an area of negative pressure that causes air to rush in. (There is one ventilator that works on negative pressure, which you’ve probably heard of: the iron lung.)
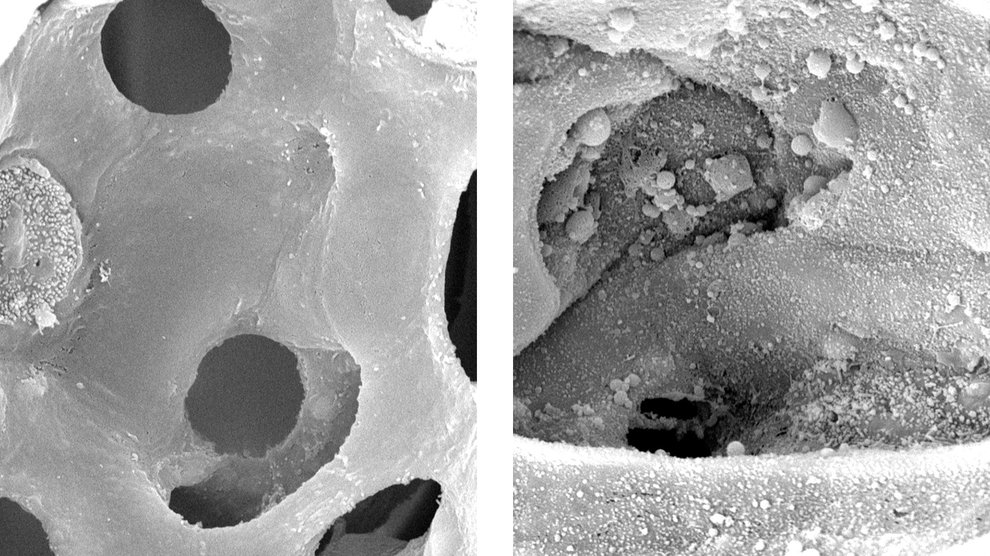
High-magnification images show the surface of alveoli in healthy mouse lungs (left) and lungs with ventilator-induced damage (right). Image Credit: Kate Hamlington Smith, University of Colorado School of Medicine
One reason COVID-19 patients need to use ventilators is because their lungs become so stiff that the diaphragm isn’t strong enough to complete its normal movement, causing breathing to slow or stop. Ventilation also keeps the lungs inflated while they heal. That’s important because inflamed capillaries in sick lungs can leak a protein-rich fluid, increasing surface tension in the liquid that normally coats the lungs and making them vulnerable to collapse.
But even healthy lungs are at risk during this process, because their tissues are not usually subject to positive pressure. That means that getting pressure levels wrong during ventilation can be dangerous. Too low, and a patient doesn’t get enough oxygen. Too high, and the lungs can become overinflated, causing their tissue to tear.
At first glance, the ventilator used in the most severe COVID-19 cases looks fairly simple: a tube that goes down the patient’s throat, two hoses that connect the tube to the machine itself (one for pushing air into the lungs and one for bringing carbon dioxide back out of the body); seals, valves, and filters to keep gases moving in the right direction; and a central case. But inside that case lives a much more complicated device, replete with pressure, flow, and oxygen sensors, and sets of circuitry and alarms associated with each element. A standard hospital ventilator has 1,500 parts, features several layers of fail-safes, and can cost around $30,000.
“The number of safeties that have to be on a medical device like this is amazing,” says Nevan Hanumara, a research scientist in MIT’s Precision Engineering Research Group. “This is second only to aerospace.”

A medical ventilator. Image Credit: Nenov, iStock
Why isn’t ventilator sharing more common?
Ventilators have such complicated inner workings in part because ventilation is much more involved than just turning on a hose. The process requires doctors to consider myriad disease factors and patient measurements, making it almost an art rather than a science. "Tidal volume," for example, refers to the amount of air in each breath, "resistance" to the ease with which air moves through the lungs, and "compliance" to how stiff or flexible the lung tissue itself is. Clinicians can also adjust how fast patients breathe and regulate air pressure at each stage of those breaths.
All this means that while setting up coventilation is relatively simple—in her YouTube video, Babcock simply uses a cheap plastic adaptor to make space for more hoses—that doesn’t necessarily mean it’s safe. The first problem, Hanumara points out, is that coventilating requires using the same pressure with two very different sets of lungs. The healthier lungs receive more air because they inflate more easily, while the sicker, less flexible lungs won’t get as much.
Secondly, he adds, sensors calibrated for one person’s measurements may not work for two, meaning the appropriate alarms might not be triggered if there is an emergency. Some COVID-19 patients, for example, experience sudden, catastrophic changes in their lung health; without alarms, it’s much more difficult to catch these changes in time. And finally there’s the matter of cross-contamination. Although two coronavirus patients sharing a ventilator can’t give each other their infections, they might still swap pneumonia microbes, or even tuberculosis.
Given these risks, research on coventilation has divided the respiratory care community. Among the more recent studies, Assistant Director of Research at SUNY Downstate Department of Emergency Medicine Lorenzo Paladino successfully coventilated four sheep for 12 hours in 2008. (Garrone, the Italian doctor, looked to that study when setting up his coventilated patients in March.) Paladino and his coauthors chose sheep for their study because adults have similar respiratory physiology and weight as humans, and aimed for 12 hours because emergency protocols allow for delivery of equipment from the Strategic National Stockpile anywhere in the continental US within that time.
The study was prompted by the 2001 anthrax attacks and 2003 SARS outbreak, Paladino says, and was meant to provide a stopgap “bridge” measure for emergency physicians with inadequate supplies waiting for backup in a disaster situation—not to replace single ventilation in the long term. Before COVID-19, the technique was most famously used after the 2017 Las Vegas concert shooting, when a single ER saw a huge surge of gunshot patients and coventilated them to keep them alive while they waited for surgery.
Paladino compares the technique to a life vest. “We don’t condone crossing the Atlantic in a life vest,” he says. “But if I’m in the middle of the Atlantic, I would rather have a life vest than not. And I would hope that a boat is coming to pick me up soon.”
The future of coventilation
Not every patient is a good candidate for coventilating, Paladino stresses. Patients with active asthma should be excluded, as should those who tend to “fight” the ventilator, trying to draw a breath when the machine is expelling air, or vice versa. But even with these caveats in place, in the wake of the COVID-19 pandemic, six major organizations—including heavyweights like the Society of Critical Care Medicine and the American Society of Anesthesiologists—have made statements against coventilating, judging it too risky and ethically questionable to be worth considering. “There’s a very legitimate concern that instead of saving two people, you just highly increased the risk of mortality for two people,” says Bradford Smith, a biomedical engineer at the University of Colorado Anschutz Medical Campus.
These serious risks point to the urgency of the recent situations in Italy and New York that have led doctors to try coventilation. Smith, who recently published a “preprint” (a not-yet-peer-reviewed preliminary study) suggesting an algorithm to match patients for safer coventilation, runs down the list of options he would try before resorting to the technique: fixing old, broken ventilators; using available machines normally used for surgical anesthesia; attaching endotracheal tubes to similar but less-invasive machines used for sleep apnea. “This is so rife with problems that the first time I heard about it, I thought, 'This is the stupidest thing I’ve ever heard,’” Smith says. “But people are taking steps to mitigate all those issues.”
Coventilating practitioners can use filters between patients to help prevent cross-contamination, for example. And protocols drawn up by Columbia Presbyterian and the Department of Health and Human Services (HHS) this spring suggest workarounds to allow for some adjustment of ventilator settings, better monitoring of both patients, and use of some built-in alarms.
As in Paladino’s case, most research on coventilation stems from a drive to prepare for the worst. Smith says he was initially inspired to work on his algorithms because he was afraid he would have to use them. (“With the news coming out of Italy, I was on these chain emails of critical care physicians, and things sounded pretty dire,” he says.) And the fact that HHS thought it necessary to convene a taskforce in Washington D.C.—which included Paladino and Babcock—to produce coventilation guidelines for future use speaks to the severity of both the pandemic and predictions for global health over the next two years.
Smith hasn’t had to use his algorithms, but he fears fall flu season may provide another opportunity. He also wonders if they may be of use in other places around the world where ventilator supplies are meager, to give physicians and respiratory therapists valuable context about how different types of patients may react to coventilation.
“This is not how nations, or even states, deal with a ventilator problem,” Paladino says. Instead, he sees coventilation playing an important role for communities that are rural or isolated, or lack access to medical care. Imagine a small hospital that owns just three ventilators and then receives six desperately sick COVID-19 patients in one night. Then what? “One night you see a spike, and you ask for help from the neighbors,” he says. In the meantime, coventilating just might keep those patients alive.
Stream Decoding COVID-19 starting on Wednesday, May 13 at 7/6c on the PBS Video app or online to learn more:



